Product Center
-
● Automatic inspection machine for particulate matter in ampoules
-
● Automatic inspection machine for oral liquid foreign objects
-
● Automatic inspection machine for lyophilized preparations in vials
-
● Automatic foreign object inspection machine for plastic bottle intravenous infusions
-
● Electronic microporous leak detector for ampoule injections
-
● Fully automatic electronic microporous leak detector for infusion
-
● Automatic foreign object inspection machine for glass bottle intravenous infusions
-
● In-line plastic ampoule electronic micro-hole leak detector
JKZ400/25 Ampoule Lyophilizer Automatic Inspection Machine
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Category
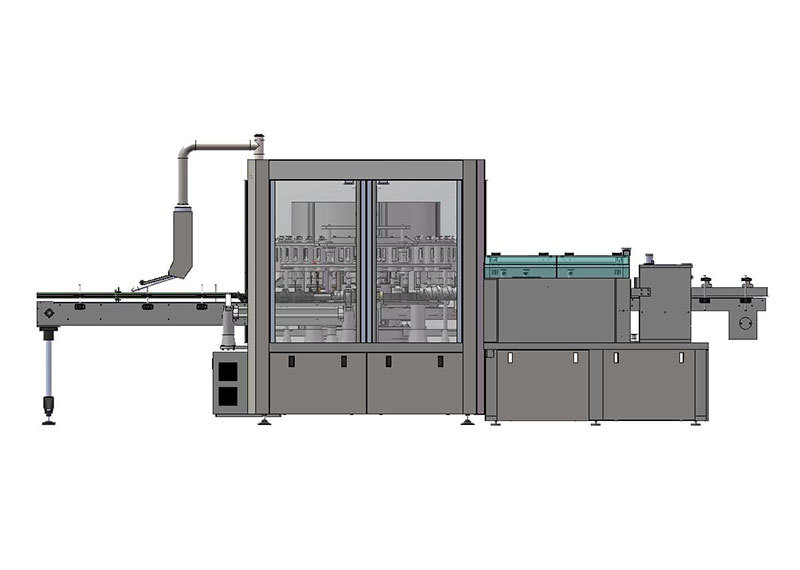
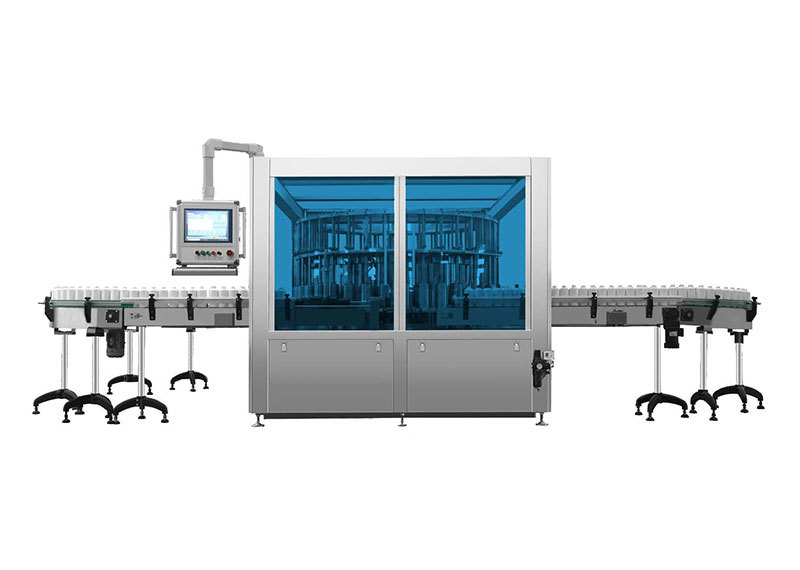
Automatic inspection machine for lyophilized preparations in vials

Details
Main Uses
This machine is designed for the detection of visible foreign objects and visual defects in lyophilized injections packaged in molded or molded-and-sealed vials at pharmaceutical factories.
How it works
The device is divided into the following main components: bottle-infeed conveyor mechanism, transition cam mechanism, rotary table inspection unit, camera-light source tracking system, bottle-outfeed cam separation mechanism, servo motor control system, and the machine body along with its protective enclosure.
Once the pharmaceutical product enters the conveyor belt, it is moved by the horizontal conveyor to the bottle-in-feed indexing wheel. The indexing wheel evenly spaces the products apart before continuously feeding them into the corresponding rotary bottle-holder stations within the inspection zone. At each station, a dedicated pressing head securely holds the product in place, ensuring that the pressing head, the product being inspected, and the rotary holder form an independent detection unit. As the turntable rotates at a constant speed, the inspected products complete more than one full rotation while passing through the inspection area. Simultaneously, a high-speed camera captures images of each product for detailed analysis. The inspection process includes six distinct stations: the cap-crimping defect detection station, the bottle-bottom and visible-drug-defect detection station, and four additional stations focused on inspecting the bottle body and drug-related defects. If any defect is detected during the inspection, the system performs a multi-frame image comparison to accurately determine the issue. Finally, when the product reaches the bottle-out-feed indexing wheel, the corresponding pressing head releases it via a cam mechanism, allowing the inspected item to move smoothly onto the outgoing wheel. From there, a rejection device separates the合格品 (qualified products) from the不合格品 (non-qualified products), ensuring only flawless items proceed further in the production line.
Testing Items
Including inspections for product defects, container appearance flaws, and sealing imperfections, the key inspection items are as follows:
1. Bottle Cap Section: Check whether the aluminum-aluminum composite cap is intact, and ensure the crimping area is uniform and aesthetically pleasing (looking for defects such as crooked crimps, burrs, wrinkles, or other imperfections).
2. Check at the bottleneck for any rubber stoppers;
3. Are the freeze-drying spots on the transparent upper part of the bottle caused by medication droplets splashing?
4. Check whether the freeze-drying process in the lower section of the vial—where the drug is located—is uniform, whether there are any areas where freeze-drying is incomplete, and whether visible foreign particles are present on the freeze-dried surface.
5. Is the freeze-drying uniform and complete at the bottom of the bottle, and are there any visible foreign particles on the freeze-dried surface?
6. Are there any visible foreign particles in the freeze-dried drug area inside the vial?
7. Are there any cracks visible on the bottle body, bottom, or other exposed areas?
8. Overfilled, underfilled, or empty bottles;
Adapting to the Environment
Ordinary Clean Work Environment
Main Technical Parameters
1. Scope of Application: Lyophilized injections in molded or controlled-type vials, 2–25 mL
2. Detection speed: 400 × (1 ± 5%) tubes/minute
3. Motors and Power: Servo motors: 0.4–2 kW; 11 units
4. Capacitance: 9 kW, 380 (1±5%) V, three-phase AC, 50 Hz
5. Workbench height: 900mm
6. Overall dimensions: 3620 × 1810 × 2000 mm (L × W × H)
7. Net Weight: Approximately 2600 kg
Main Technical Features
1. Detection accuracy is adjustable, catering to the varying inspection requirements of different projects.
2. The conveying system, designed with patented technology (see "Patented Technologies" under "Manufacturing & Quality" on the company website), ensures smooth and seamless transport of the objects being inspected.
3. Multiple components now use servo motor control instead of traditional mechanical transmission, enabling seamless integration between control and inspection programs, thereby enhancing the precision requirements for inspected items.
4. Standard components feature a quick-release locating pin design, making component replacement simple and adjustments convenient.
5. Equipped with a high-resolution, imported industrial camera offering 50x magnification, this system illuminates the object being inspected directly, enhancing the visibility of defects and delivering detection accuracy and results that surpass those achieved through manual inspection.
6. Up to six workstation inspection stations can detect all types of defects.
7. Key components of the equipment are all manufactured using precision CNC machines, ensuring their accuracy and quality.
8. The entire machine boasts a sleek and elegant design, with special protective plastic coating applied to components such as the machine body plate and baseplate.
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Main Uses
This machine is designed for the detection of visible foreign objects and visual defects in lyophilized injections packaged in molded or molded-and-sealed vials at pharmaceutical factories.
How it works
The device is divided into the following main components: bottle-infeed conveyor mechanism, transition cam mechanism, rotary table inspection unit, camera-light source tracking system, bottle-outfeed cam separation mechanism, servo motor control system, and the machine body along with its protective enclosure.
Once the pharmaceutical product enters the conveyor belt, it is moved by the horizontal conveyor to the bottle-in-feed indexing wheel. The indexing wheel evenly spaces the products apart before continuously feeding them into the corresponding rotary bottle-holder stations within the inspection zone. At each station, a dedicated pressing head securely holds the product in place, ensuring that the pressing head, the product being inspected, and the rotary holder form an independent detection unit. As the turntable rotates at a constant speed, the inspected products complete more than one full rotation while passing through the inspection area. Simultaneously, a high-speed camera captures images of each product for detailed analysis. The inspection process includes six distinct stations: the cap-crimping defect detection station, the bottle-bottom and visible-drug-defect detection station, and four additional stations focused on inspecting the bottle body and drug-related defects. If any defect is detected during the inspection, the system performs a multi-frame image comparison to accurately determine the issue. Finally, when the product reaches the bottle-out-feed indexing wheel, the corresponding pressing head releases it via a cam mechanism, allowing the inspected item to move smoothly onto the outgoing wheel. From there, a rejection device separates the合格品 (qualified products) from the不合格品 (non-qualified products), ensuring only flawless items proceed further in the production line.
Testing Items
Including inspections for product defects, container appearance flaws, and sealing imperfections, the key inspection items are as follows:
1. Bottle Cap Section: Check whether the aluminum-aluminum composite cap is intact, and ensure the crimping area is uniform and aesthetically pleasing (looking for defects such as crooked crimps, burrs, wrinkles, or other imperfections).
2. Check at the bottleneck for any rubber stoppers;
3. Are the freeze-drying spots on the transparent upper part of the bottle caused by medication droplets splashing?
4. Check whether the freeze-drying process in the lower section of the vial—where the drug is located—is uniform, whether there are any areas where freeze-drying is incomplete, and whether visible foreign particles are present on the freeze-dried surface.
5. Is the freeze-drying uniform and complete at the bottom of the bottle, and are there any visible foreign particles on the freeze-dried surface?
6. Are there any visible foreign particles in the freeze-dried drug area inside the vial?
7. Are there any cracks visible on the bottle body, bottom, or other exposed areas?
8. Overfilled, underfilled, or empty bottles;
Adapting to the Environment
Ordinary Clean Work Environment
Main Technical Parameters
1. Scope of Application: Lyophilized injections in molded or controlled-type vials, 2–25 mL
2. Detection speed: 400 × (1 ± 5%) tubes/minute
3. Motors and Power: Servo motors: 0.4–2 kW; 11 units
4. Capacitance: 9 kW, 380 (1±5%) V, three-phase AC, 50 Hz
5. Workbench height: 900mm
6. Overall dimensions: 3620 × 1810 × 2000 mm (L × W × H)
7. Net Weight: Approximately 2600 kg
Main Technical Features
1. Detection accuracy is adjustable, catering to the varying inspection requirements of different projects.
2. The conveying system, designed with patented technology (see "Patented Technologies" under "Manufacturing & Quality" on the company website), ensures smooth and seamless transport of the objects being inspected.
3. Multiple components now use servo motor control instead of traditional mechanical transmission, enabling seamless integration between control and inspection programs, thereby enhancing the precision requirements for inspected items.
4. Standard components feature a quick-release locating pin design, making component replacement simple and adjustments convenient.
5. Equipped with a high-resolution, imported industrial camera offering 50x magnification, this system illuminates the object being inspected directly, enhancing the visibility of defects and delivering detection accuracy and results that surpass those achieved through manual inspection.
6. Up to six workstation inspection stations can detect all types of defects.
7. Key components of the equipment are all manufactured using precision CNC machines, ensuring their accuracy and quality.
8. The entire machine boasts a sleek and elegant design, with special protective plastic coating applied to components such as the machine body plate and baseplate.
Next Page
Next Page
Related Products
Inquiry