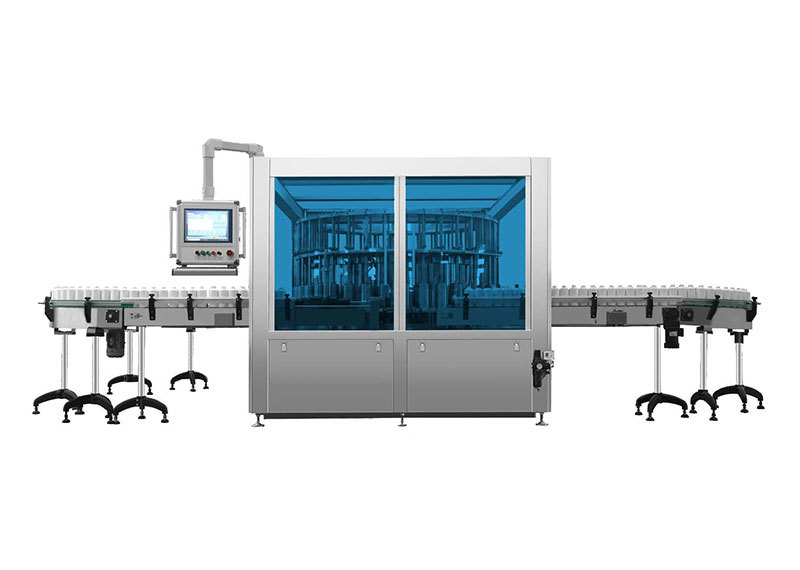
Product Center
-
● Automatic inspection machine for particulate matter in ampoules
-
● Automatic inspection machine for oral liquid foreign objects
-
● Automatic inspection machine for lyophilized preparations in vials
-
● Automatic foreign object inspection machine for plastic bottle intravenous infusions
-
● Electronic microporous leak detector for ampoule injections
-
● Fully automatic electronic microporous leak detector for infusion
-
● Automatic foreign object inspection machine for glass bottle intravenous infusions
-
● In-line plastic ampoule electronic micro-hole leak detector
KLJ300 Vial Vacuum Inspection Machine
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Category
Vacuum leak detector for vials
Keyword

Details
Main Uses
Primarily used for leak testing of lyophilized preparations in vials, powder-injectable formulations, and other sealed products, it mainly checks whether the levels of residual oxygen and moisture in the headspace inside the container meet the required standards.
How it works
As shown in Figure 1: The drug to be inspected is conveyed via the bottle-infeed conveyor belt (1) to the bottle-infeed indexing wheel (2); the bottle-infeed indexing wheel then transfers the drugs onto the inspection indexing wheel (4). During this process, the inspection indexing wheel passes two laser detection units (3), which measure the headspace oxygen and moisture levels inside the containers. Based on the analysis and calculation of the detection data, a pass/fail determination is made. According to the results from the laser detection units, commands are issued: qualified products are directed by the bottle-outfeed indexing wheel (5), while defective items are routed by the bottle-outfeed indexing wheel (6) into separate collection trays.

Figure 1
1. Bottle-feeding mesh belt
2. Bottle-feeding cam
3. Laser Detection Device
4. Detection Dial
5. Bottle-discharging cam
6. Bottle-discharging cam
Detection Principle
Utilize the principle of laser absorption spectroscopy to detect the oxygen and moisture content in the product headspace, thereby determining whether the product meets quality standards.
Using two laser diodes that emit lasers with wavelengths matching the absorption peaks of the target molecules (oxygen and water), the lasers are directed through the headspace of the container to reach the detector. The system then obtains measurement results by employing precise adjustment, computational, and signal-processing techniques, compares these results against established quality standards, and determines whether the product meets specifications (see Figure 2).

Figure 2 (Fig-2)
Main Technical Parameters
1. Scope of Application: Lyophilized preparations in glass vials, powder-for-injection formulations, 1 mL to 20 mL
2. Detection Speed: 1–300 bottles/min (1–10 mL); 1–250 bottles/min (20 mL)
3. Capacitance: AC 380V, 50Hz, 3kW
4. Overall Dimensions: 3600mm x 1400mm x 1800mm (Length × Width × Height)
5. Net Weight: Approximately 1500 kg
Product Features
1. Fully automatic online inspection, capable of operating both as a standalone unit or integrated into a production line.
2. Will not damage the container being tested or the medication.
3. Detection is performed using the principle of laser absorption spectroscopy, ensuring no secondary contamination of the pharmaceuticals.
4. Highly versatile. By simply replacing the standard components, it can be used to inspect containers of various volume specifications.
5. A human-machine-friendly touchscreen interface featuring powerful functions such as control, display, data acquisition, storage, and printing;
6. Has obtained multiple patents (see "Patented Technologies" under "Manufacturing & Quality" on the company website)
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Main Uses
Primarily used for leak testing of lyophilized preparations in vials, powder-injectable formulations, and other sealed products, it mainly checks whether the levels of residual oxygen and moisture in the headspace inside the container meet the required standards.
How it works
As shown in Figure 1: The drug to be inspected is conveyed via the bottle-infeed conveyor belt (1) to the bottle-infeed indexing wheel (2); the bottle-infeed indexing wheel then transfers the drugs onto the inspection indexing wheel (4). During this process, the inspection indexing wheel passes two laser detection units (3), which measure the headspace oxygen and moisture levels inside the containers. Based on the analysis and calculation of the detection data, a pass/fail determination is made. According to the results from the laser detection units, commands are issued: qualified products are directed by the bottle-outfeed indexing wheel (5), while defective items are routed by the bottle-outfeed indexing wheel (6) into separate collection trays.

Figure 1
1. Bottle-feeding mesh belt
2. Bottle-feeding cam
3. Laser Detection Device
4. Detection Dial
5. Bottle-discharging cam
6. Bottle-discharging cam
Detection Principle
Utilize the principle of laser absorption spectroscopy to detect the oxygen and moisture content in the product headspace, thereby determining whether the product meets quality standards.
Using two laser diodes that emit lasers with wavelengths matching the absorption peaks of the target molecules (oxygen and water), the lasers are directed through the headspace of the container to reach the detector. The system then obtains measurement results by employing precise adjustment, computational, and signal-processing techniques, compares these results against established quality standards, and determines whether the product meets specifications (see Figure 2).

Figure 2 (Fig-2)
Main Technical Parameters
1. Scope of Application: Lyophilized preparations in glass vials, powder-for-injection formulations, 1 mL to 20 mL
2. Detection Speed: 1–300 bottles/min (1–10 mL); 1–250 bottles/min (20 mL)
3. Capacitance: AC 380V, 50Hz, 3kW
4. Overall Dimensions: 3600mm x 1400mm x 1800mm (Length × Width × Height)
5. Net Weight: Approximately 1500 kg
Product Features
1. Fully automatic online inspection, capable of operating both as a standalone unit or integrated into a production line.
2. Will not damage the container being tested or the medication.
3. Detection is performed using the principle of laser absorption spectroscopy, ensuring no secondary contamination of the pharmaceuticals.
4. Highly versatile. By simply replacing the standard components, it can be used to inspect containers of various volume specifications.
5. A human-machine-friendly touchscreen interface featuring powerful functions such as control, display, data acquisition, storage, and printing;
6. Has obtained multiple patents (see "Patented Technologies" under "Manufacturing & Quality" on the company website)
Related Products
Inquiry