Product Center
-
● Automatic inspection machine for particulate matter in ampoules
-
● Automatic inspection machine for oral liquid foreign objects
-
● Automatic inspection machine for lyophilized preparations in vials
-
● Automatic foreign object inspection machine for plastic bottle intravenous infusions
-
● Electronic microporous leak detector for ampoule injections
-
● Fully automatic electronic microporous leak detector for infusion
-
● Automatic foreign object inspection machine for glass bottle intravenous infusions
-
● In-line plastic ampoule electronic micro-hole leak detector
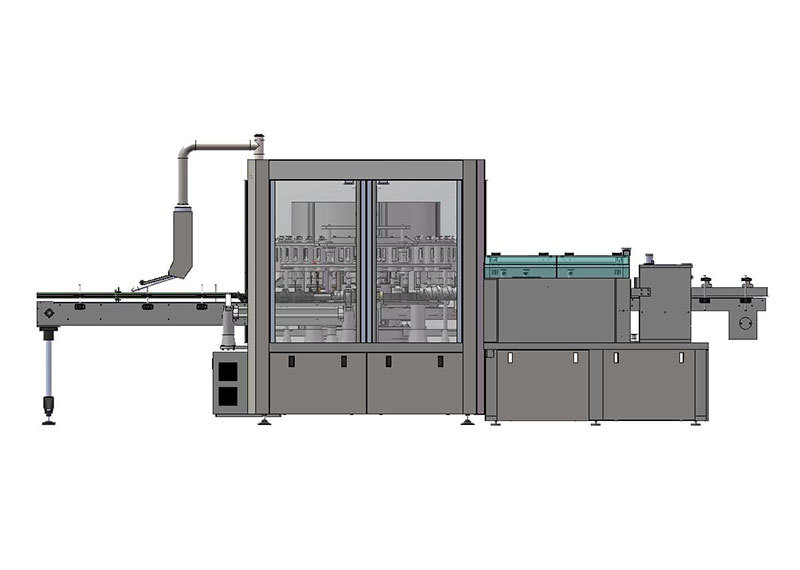
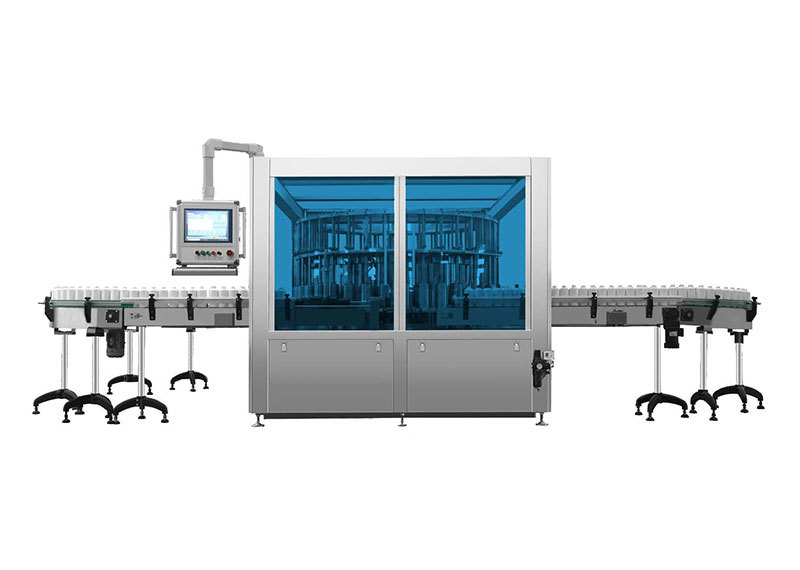
JSSZ/300 Plastic Bottle Large Infusion Foreign Object Automatic Inspection Machine
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Category
Automatic foreign object inspection machine for plastic bottle intravenous infusions

Details
Main Uses
Primarily used for the automated inspection of foreign objects in large-volume plastic IV solutions produced at pharmaceutical plants. Inspection items include foreign particles such as glass shards, hair, white flakes, and white spots, as well as liquid-level detection. Detection range: 50ml–250ml plastic and glass bottles for large-volume IV solutions.
Technical Specifications
1. Detection speed: 0–300 bottles/min
2. Compatibility Specifications: 50–250ml large-volume infusion plastic or glass bottles
Key Features
1. The entire machine features a compact structure, simple design, convenient maintenance, and strong versatility.
2. The testing equipment is positioned above the workbench, meeting GMP requirements.
3. Detection accuracy is adjustable to meet pharmaceutical manufacturers' production requirements.
4. The detection data can be retrieved, reviewed, and stored, and it features a print interface. This ensures process parameters are traceable and meet GMP requirements.
5. Major key components, such as cameras, light sources, servo motors, main motors, frequency converters, proximity switches, controllers, transmission bearings, and plain bearings, are sourced from either imported or well-known domestic brands.
6. The test procedure, recognized by the European Pharmacopoeia and the U.S. FDA—known as the Knapp test—ensures that all key functions and parameters are verifiable.
Mainly engaged in the research and development of ceramic new material valves, with metal valves (gate valves, globe valves, ball valves, butterfly valves) as a secondary business. Ceramic valves have been successfully applied in many new energy battery material plants, thermal power plants, nuclear power plants, chemical plants, steel plants, coal chemical industry, polysilicon and other industries, and have been listed as special-purpose products, contributing to energy conservation and environmental protection for enterprises and society.
Main Uses
Primarily used for the automated inspection of foreign objects in large-volume plastic IV solutions produced at pharmaceutical plants. Inspection items include foreign particles such as glass shards, hair, white flakes, and white spots, as well as liquid-level detection. Detection range: 50ml–250ml plastic and glass bottles for large-volume IV solutions.
Technical Specifications
1. Detection speed: 0–300 bottles/min
2. Compatibility Specifications: 50–250ml large-volume infusion plastic or glass bottles
Key Features
1. The entire machine features a compact structure, simple design, convenient maintenance, and strong versatility.
2. The testing equipment is positioned above the workbench, meeting GMP requirements.
3. Detection accuracy is adjustable to meet pharmaceutical manufacturers' production requirements.
4. The detection data can be retrieved, reviewed, and stored, and it features a print interface. This ensures process parameters are traceable and meet GMP requirements.
5. Major key components, such as cameras, light sources, servo motors, main motors, frequency converters, proximity switches, controllers, transmission bearings, and plain bearings, are sourced from either imported or well-known domestic brands.
6. The test procedure, recognized by the European Pharmacopoeia and the U.S. FDA—known as the Knapp test—ensures that all key functions and parameters are verifiable.
Related Products
Inquiry